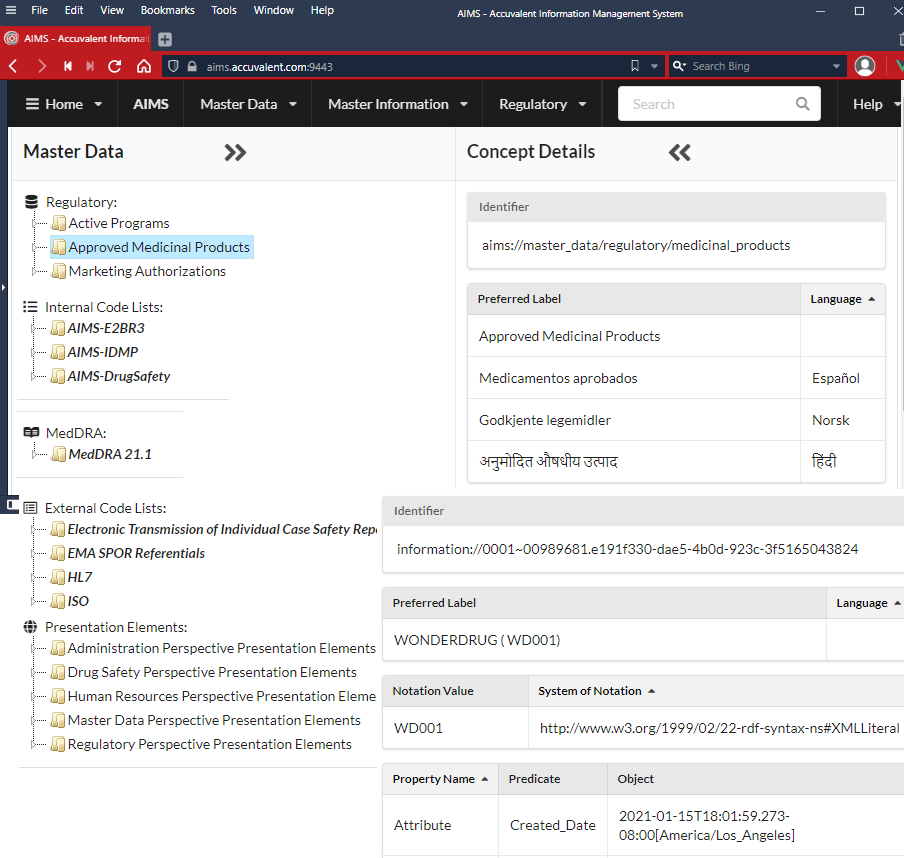
Centrally manage R&D master data concepts and allow queries by disparate business processes and analytical engines.
Include key reference information like ‘Approved Medicinal Products’ dynamically in master data collections e.g. by their lifecycle states.
Import code lists for standards like E2BR3, IDMP from external sources e.g. EMA SPOR, HL7, ISO and be available to business processes and analytical engines.
Create and maintain internal code lists for business processes.
Support standard hierarchical dictionaries like MedDRA.
Standardize any administrative data element required for perspectives.
Multi language support across all Master Data Concepts.
